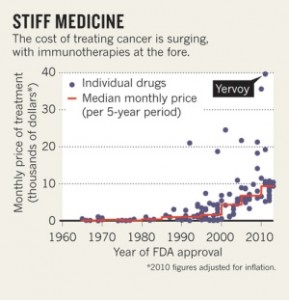
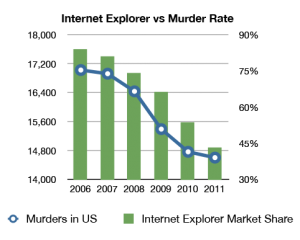
Two hundred and sixty six years ago today, James Lind began what is regarded as the first proper controlled clinical trial
On the 20th May, 1747, I took twelve patients in the scurvy on board the Salisbury at sea. Their cases were as similar as I could have them. They all in general had putrid gums, the spots and lassitude, with weakness of their knees. They lay together in one place, being a proper apartment for the sick in the fore-hold; and had one diet in common to all, viz., water gruel sweetened with sugar in the morning; fresh mutton broth often times for dinner; at other times puddings, boiled biscuit with sugar etc.; and for supper barley, raisins, rice and currants, sago and wine, or the like. Two of these were ordered each a quart of cyder a day. Two others took twenty five gutts of elixir vitriol three times a day upon an empty stomach, using a gargle strongly acidulated with it for their mouths. Two others took two spoonfuls of vinegar three times a day upon an empty stomach, having their gruels and their other food well acidulated with it, as also the gargle for the mouth. Two of the worst patients, with the tendons in the ham rigid (a symptom none the rest had) were put under a course of sea water. Of this they drank half a pint every day and sometimes more or less as it operated by way of gentle physic. Two others had each two oranges and one lemon given them every day. These they eat with greediness at different times upon an empty stomach. They continued but six days under this course, having consumed the quantity that could be spared. The two remaining patients took the bigness of a nutmeg three times a day of an electuray recommended by an hospital surgeon made of garlic, mustard seed, rad. raphan., balsam of Peru and gum myrrh, using for common drink narley water well acidulated with tamarinds, by a decoction of wich, with the addition of cremor tartar, they were gently purged three or four times during the course.
The consequence was that the most sudden and visible good effects were perceived from the use of the oranges and lemons; one of those who had taken them being at the end of six days fit four duty. The spots were not indeed at that time quite off his body, nor his gums sound; but without any other medicine than a gargarism or elixir of vitriol he became quite healthy before we came into Plymouth, which was on the 16th June. The other was the best recovered of any in his condition, and being now deemed pretty well was appointed nurse to the rest of the sick …
Lind knew very little about scurvy apart from the typical progress of the disease, and he had no real idea of how the treatments might work. That’s a handicap in coming up with ideas for treatment, but not in doing fair tests of whether treatments work.
The trial didn’t have an untreated group: all the patients got one of the treatments recommended by experts. There’s no need for a controlled trial to have an untreated group — if there is an existing treatment, you want to compare to that treatment; if there is none, you may want to compare immediate vs delayed treatment.
Despite the dramatic success of fruit juice in the trial, it wasn’t adopted as a treatment. That, sadly, can still be the case today. New drugs or surgical techniques are taken up enthusiastically, but boring interventions like nurse home visits or surgery checklists get less attention. Still, things are much better than they were even twenty years ago. Nearly all of medicine accepts the idea of randomized controlled comparison, and it is spreading to other areas such as development economics.
There are two excellent, free books about clinical trials and health choices: Testing Treatments, from the James Lind Initiative, and Smart Health Choices, from Les Irwig (at Sydney Uni) and coworkers.
Much of clinical trials development is unapologetically technical, but there are important areas where public participation can help:
- The James Lind Alliance asks patients and clinicians to say what questions matter most. Clinical trials still tend to answer questions that are scientifically interesting or commercially important, not what actually matters most to patients
- The Cochrane Collaboration is attempting to collect and summarise all randomized trials. The Cochrane Consumers Network is for non-specialist participation — in particular as Consumer Referees to help ensure that summaries of research address the questions that are important to consumers and are presented in language that consumers can understand.
- Ethics Committees that review clinical trials and other human research in New Zealand are required to have non-specialist community members. This is a substantial commitment, but one that is important if ethics committees are to be more than just red tape.
- And if you haven’t yet signed the AllTrials petition, calling for the results of all clinical trials to be published so we can know what treatments work, now would be an excellent time.