Publicise it while it still works?
This is from The Times. In fact, per the BBC, it’s the leading story on the front page of tomorrow’s Times, above Brexit.
The drug in question, aducanumab, had promising and widely reported early results. It was less widely reported that the definitive clinical trials were stopped early in March of this year, when it wasn’t doing any good. The decision to stop was based on data collected through last December; after stopping, more data has rolled in. Biogen, the makers of the drug, are now claiming that the data accumulated since then show a benefit at the highest dose; the FDA have given them permission to apply for approval. Here’s what has happened to their stock price
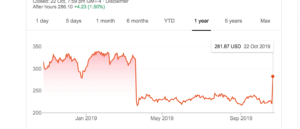
So far, no detailed data or analyses have been published. The New York Times story, which is a lot more chill, says that the results will be presented at a conference in December. A working drug would be genuinely important news, but putting it on the front page right now would be a bit premature even in a slow news week.
There’s a general pattern with drug breakthrough news, where the good news is presented with big headlines in the main body of the paper, and the bad news that walks it back is in the business section, with small headlines. One long-term result is a misleadingly optimistic public view of new drugs, most of which aren’t breakthroughs.
Update: note that the post-good-news stock price jump was a lot smaller than the post-bad-news collapse, and the price fell over the day after the peak — the stock markets aren’t betting on this being a spectacularly successful drug.
Thomas Lumley (@tslumley) is Professor of Biostatistics at the University of Auckland. His research interests include semiparametric models, survey sampling, statistical computing, foundations of statistics, and whatever methodological problems his medical collaborators come up with. He also blogs at Biased and Inefficient See all posts by Thomas Lumley »
