Unpublished clinical trials
We’ve known since at least the 1980s that there’s a problem with clinical trial results not being published. Tracking the non-publication rate is time-consuming, though. There’s a new website out that tries to automate the process, and a paper that claims it’s fairly accurate, at least for the subset of trials registered at ClinicalTrials.gov. It picks up most medical journals and also picks up results published directly at ClinicalTrials.gov — an alternative pathway for boring results such as dose equivalence studies for generics.
Here’s the overall summary for all trial organisers with more than 30 registered trials:
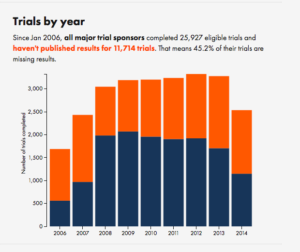
The overall results are pretty much what people have been claiming. The details might surprise you if you haven’t looked into the issue carefully. There’s a fairly pronounced difference between drug companies and academic institutions — the drug companies are better at publishing their trials.
For example, compare Merck to the Mayo Clinic
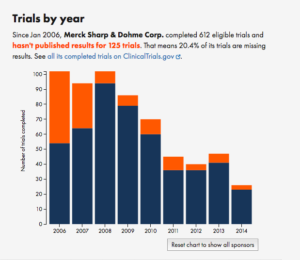
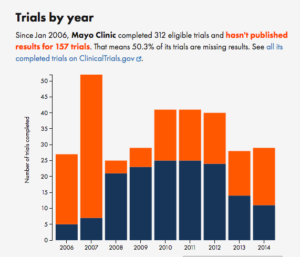
It’s not uniform, but the trend is pretty clear.
Thomas Lumley (@tslumley) is Professor of Biostatistics at the University of Auckland. His research interests include semiparametric models, survey sampling, statistical computing, foundations of statistics, and whatever methodological problems his medical collaborators come up with. He also blogs at Biased and Inefficient See all posts by Thomas Lumley »