The Guardian reports “Gen Z males twice as likely as baby boomers to believe wives should obey husbands”, with similar phrasing in headlines from the Daily Mail, and the NY Post, and in the lede from the BBC. This has, unsurprisingly, caused a bit of concern.
Looking at the original information from King’s College London (research for International Women’s Day†) the trend seems to go over all ages
This seems strange. I would not have thought women’s equality had been getting steadily worse for the past eighty years. Do we just have a bad question, or a bad sample, or what? The page at King’s College shows broadly similar patterns for other gender attitude questions, though often less extreme. It’s not just the question but it might be partly the question. In particular, there might be a carryover from ‘obey’ in wedding vows, which is not quite the same. However, “A husband should have the final word on important decisions made in his home” gets very similar answers to the “obey” question.
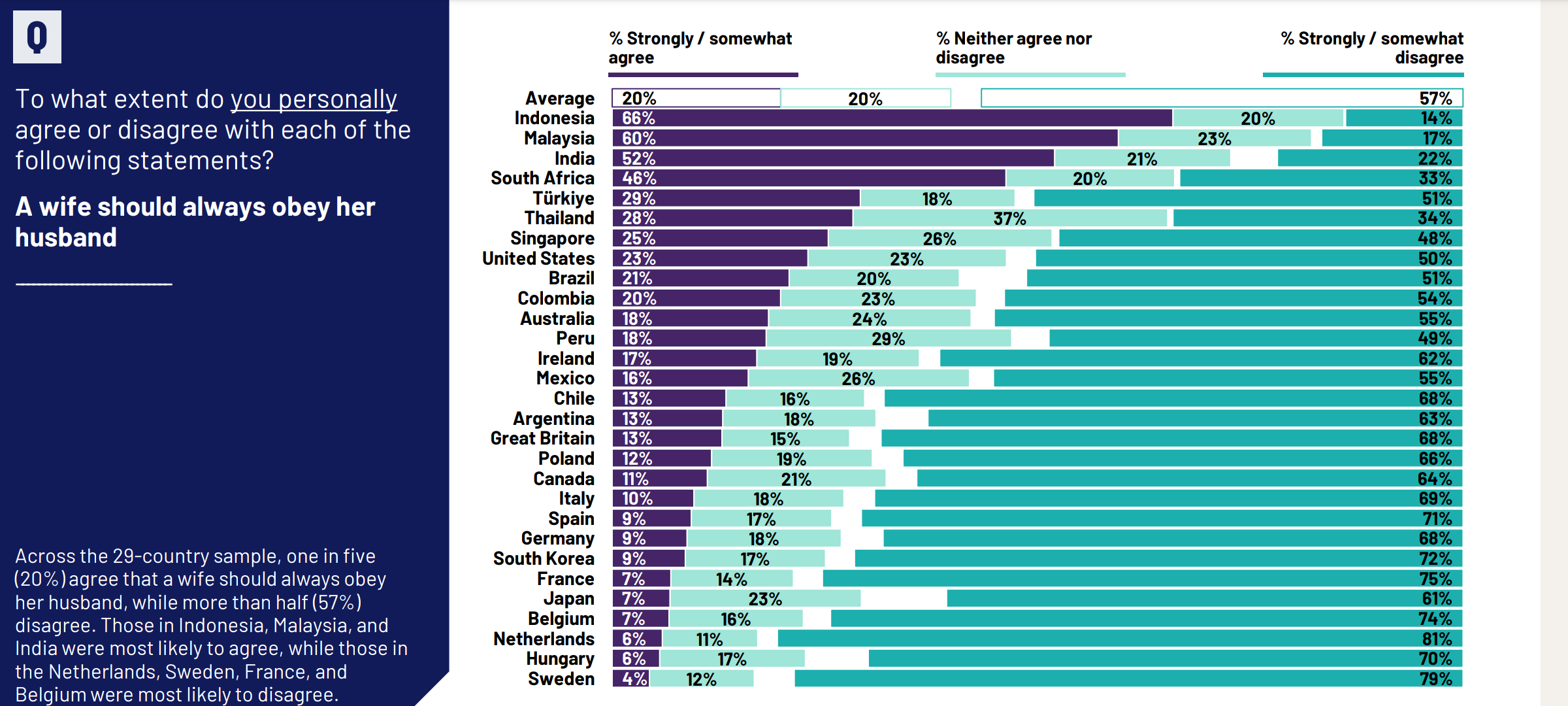
Here’s the worldwide comparison for the ‘obey’ question:
There’s a huge amount of variation between countries, so the results will be sensitive to how countries are combined. Honest and competent researchers will give you this sort of information, and there is a full PDF report actually linked from the King’s College page, near the top! It has a Technical Note on the last page that says in part
The data is weighted so that the composition of each country’s sample best reflects the demographic profile of the adult population according to the most recent census data. “The Global Country Average” reflects the average result for all the countries and markets in which the survey was conducted. It has not been adjusted to the population size of each country or market and is not intended to suggest a total result. [emphasis added]
It would be interesting to see separate trends for countries and regions, rather than suggesting a total result, when the responses from different countries are so different.
† Yes, there is. November 19.

Recent comments